MCL Implementing Canntab Drug Development Strategy
MCL Implementing Canntab Drug Development Strategy
Sydney, Mar 29, 2018 AEST (ABN Newswire) - The board of Queensland Bauxite Limited ( ASX:QBL) (QBL) is pleased to update the market on developments in the drug development strategy for its subsidiary, Medical Cannabis Limited (MCL). MCL, in conjunction with Certara, a pharmaceutical consulting firm, have developed a drug development strategy for the distribution of Canntab XR medication in the Australian market.
ASX:QBL) (QBL) is pleased to update the market on developments in the drug development strategy for its subsidiary, Medical Cannabis Limited (MCL). MCL, in conjunction with Certara, a pharmaceutical consulting firm, have developed a drug development strategy for the distribution of Canntab XR medication in the Australian market.
The Certara team includes Dr Andreas Wallnoefer, who held a significant tenure as Global Head of Early Development at Roche and Dr Graham Scott, who prior to Certara, was European lead of Clinical Pharmacology at Takeda. The drug development team is led by Andrew Kavasilas, MCL's technical expert on cannabis.
Highlights
- The board has an effective mix of both pharmaceutical and cannabis expertise working on MCL's drug development strategy
- MCL's strategy will position Canntab as a reliable and medical based 'product of choice'
- Revenue is targeted in Australia in 2018 via Canntab sales under the Special Access Scheme
- MCL is targeting broader adoption in pain relief through the clinical trial process
Background
Canntab is a Canadian based producer and distributor of medical cannabis pharmaceuticals, targeting various medical markets via its range of Canntab XR tablets. MCL is a diversified cannabis company with the rights to distribute Canntab products in Australia and Asia via the 50/50 Canntab Australia joint venture. Certara is a consulting firm assisting MCL evaluate potential drug development pathway's for the Canntab product suite.
For more information on the MCL/Canntab joint venture and products, please see the following ASX announcement:
http://abnnewswire.net/lnk/8TY23L1J
Drug development strategy
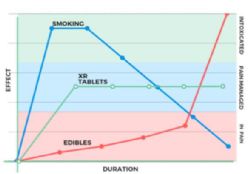
MCL's drug development strategy is to position the Canntab product as a reliable medical 'product of choice' that practitioners will be confident prescribing. Medical cannabis is still relatively difficult to access for patients in need in Australia, despite positive regulatory momentum over the last two years. Management believe the delivery mechanism of this patent pending GMP pharmaceutical grade extended release pill will be better appreciated by the regulatory and medical community compared to smoking or other delivery methods.
Initial revenue is expected to come through the special access scheme (SAS), with Canntab products expected to be imported into Australia 1H 2018. While the special access scheme is currently limited in scale, the company will continue studies for broader market penetration in tandem with the SAS. The data generated from clinical trials can be utilised near term in marketing Canntab as a reliable 'product of choice' on the SAS.
Near term, the company intends to confirm the following characteristics of Canntab XR tablets, to be tested in our own local clinical trials:
- Canntab has a consistent and accurate dosage: MCL will complete pharmacokinetic studies to compare Canntab with alternative medical cannabis preparations. The aim is to substantiate the claim that Canntab has an optimal delivery system and low volatility in the targeted levels of THC and CBD in the blood.
- Drug-drug interactions: Studies to determine the impact of the interaction of Canntab products with other drugs (e.g. alcohol).
- Efficacy in reducing opioid use: Descriptive efficacy of pain relief including reduction in co-medicines (opioids) in Chronic Non Cancer Pain (CNCP). This can help determine to what extent Canntab may be able to take market share from incumbent medications and competing medical cannabis treatments.
Management believe the Canntab pill will be competitively placed in the Australian market place given the concerns of the medical industry around the safety of smoked and vaporised cannabinoids.
Value adding milestones ahead
Management is focusing on delivery to the guidance of:
- Secure Canntab supply in 1H2018 for delivery into special access scheme
- Progress with market research program
- Progress with clinical trial applications and strategy
Comment from Pnina Feldman, Chairperson of QBL
"We are pleased to update the market on MCL's progress in preparing the Canntab product to be market ready. We believe this product is well positioned compared to competing cannabis products and will make a lasting difference to many Australians in need. 2018 is shaping up to be a milestone year and we look forward to seeing the first revenues from our medical division shortly."
To view figures, please visit:
http://abnnewswire.net/lnk/01708J8T
About Queensland Bauxite Ltd
 Queensland Bauxite Limited (ASX:QBL) is an Australian listed company focused on the exploration and development of its bauxite tenements in Queensland and New South Wales. The Company's lead project is the South Johnstone Bauxite Deposit in northern Queensland which has rail running through the project area and is approximately 15-24 kilometres from the nearest deep water port. The Company intends to become a bauxite producer with a focus on commencing production at South Johnstone as early as possible. The Company also pursues additional investment opportunities, and has agreed to acquire a 100% shareholding in Medical Cannabis Limited, an Australian leader in the hemp and Cannabis industries, and a 100% shareholding in Medcan Australia Pty Ltd, a company with an ODC cultivation and production License and a DA approved Cannabis production and manufacturing facility.
Queensland Bauxite Limited (ASX:QBL) is an Australian listed company focused on the exploration and development of its bauxite tenements in Queensland and New South Wales. The Company's lead project is the South Johnstone Bauxite Deposit in northern Queensland which has rail running through the project area and is approximately 15-24 kilometres from the nearest deep water port. The Company intends to become a bauxite producer with a focus on commencing production at South Johnstone as early as possible. The Company also pursues additional investment opportunities, and has agreed to acquire a 100% shareholding in Medical Cannabis Limited, an Australian leader in the hemp and Cannabis industries, and a 100% shareholding in Medcan Australia Pty Ltd, a company with an ODC cultivation and production License and a DA approved Cannabis production and manufacturing facility.


![abnnewswire.com]()
Related Companies
Social Media
Share this Article

 ASX:QBL) (QBL) is pleased to update the market on developments in the drug development strategy for its subsidiary, Medical Cannabis Limited (MCL). MCL, in conjunction with Certara, a pharmaceutical consulting firm, have developed a drug development strategy for the distribution of Canntab XR medication in the Australian market.
ASX:QBL) (QBL) is pleased to update the market on developments in the drug development strategy for its subsidiary, Medical Cannabis Limited (MCL). MCL, in conjunction with Certara, a pharmaceutical consulting firm, have developed a drug development strategy for the distribution of Canntab XR medication in the Australian market.  Queensland Bauxite Limited (ASX:QBL) is an Australian listed company focused on the exploration and development of its bauxite tenements in Queensland and New South Wales. The Company's lead project is the South Johnstone Bauxite Deposit in northern Queensland which has rail running through the project area and is approximately 15-24 kilometres from the nearest deep water port. The Company intends to become a bauxite producer with a focus on commencing production at South Johnstone as early as possible. The Company also pursues additional investment opportunities, and has agreed to acquire a 100% shareholding in Medical Cannabis Limited, an Australian leader in the hemp and Cannabis industries, and a 100% shareholding in Medcan Australia Pty Ltd, a company with an ODC cultivation and production License and a DA approved Cannabis production and manufacturing facility.
Queensland Bauxite Limited (ASX:QBL) is an Australian listed company focused on the exploration and development of its bauxite tenements in Queensland and New South Wales. The Company's lead project is the South Johnstone Bauxite Deposit in northern Queensland which has rail running through the project area and is approximately 15-24 kilometres from the nearest deep water port. The Company intends to become a bauxite producer with a focus on commencing production at South Johnstone as early as possible. The Company also pursues additional investment opportunities, and has agreed to acquire a 100% shareholding in Medical Cannabis Limited, an Australian leader in the hemp and Cannabis industries, and a 100% shareholding in Medcan Australia Pty Ltd, a company with an ODC cultivation and production License and a DA approved Cannabis production and manufacturing facility.