Update Investee Cortical Dynamics Developments
Perth, Aug 31, 2022 AEST (ABN Newswire) - BPH Energy Limited ( ASX:BPH) wishes to provide an update on Developments for Cortical Dynamics Ltd ("Cortical"), BPH holding 17.7%.
ASX:BPH) wishes to provide an update on Developments for Cortical Dynamics Ltd ("Cortical"), BPH holding 17.7%.
Partnership with ENCEVIS
Cortical Dynamics entered into a partnership with Austrian EEG experts ENCEVIS /AIT with a view to further enhance the BARM(TM) technology. The AIT Austrian Institute of Technology is Austria's largest research and technology organisation employing over 1,300 people. The Republic of Austria (through the Federal Ministry for Climate Protection, Environment, Energy, Mobility, Innovation and Technology) owns 50.46% of AIT, while the Federation of Austrian Industries owns the other 49.54%. ENCEVIS is a division of AIT that specialises in EEG Cortical Dynamics wins AI prestigious grant and makes appointments of Head of Data Analytics and Project Management In June 2022 Cortical Dynamics won a prestigious grant from the MTPConnect BMTH program. The matched funding that will help Cortical develop an AI and machine learning capacity for BARM(TM).
Conjunctionally Cortical has appointed a world class Head of Data Analytics who will focus on developing for the company a deep understanding of sedation level monitoring systems using Artificial Intelligence including neurophysiology (EEG), machine learning, statistical analysis, anaesthesiology. Application areas will include optimal management of anaesthesia and sedatives in the operating room and the ICU. In addition Cortical has appointed a full-time project manager.
FDA 510(K) Submission
Cortical has begun the FDA 510K filing process for BARM(TM) in the USA assisted by Washington based technical advisors MCRA. The Food and Drug Administration ("FDA") is the federal agency of the United States Department of Health and Human Services which regulates the sale of medical device products (including diagnostic tests) in the U.S. and monitors the safety of all regulated medical products. FDA approval is a necessary precursor for sales of BARM(TM) to commence in the USA.
New USA Patent
Throughout the year Cortical continued to expand its IP portfolio in the USA. Cortical has developed an extensive patent portfolio encapsulating the BARM, providing critical patent protection across several key brain monitoring markets. Cortical's competitive advantage is underpinned by a strong patent position covered by five patent families and 36 granted patents.
About Cortical Dynamics
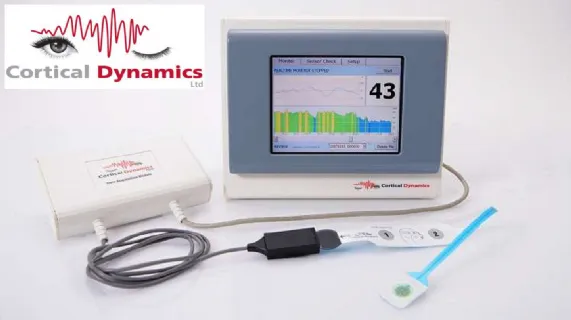
Cortical is an Australian based medical device neurotechnology company that is developing BARM(TM), an industry leading EEG (electrical activity) brain function monitor. BARM(TM) is being developed to better detect the effect of anaesthetic agents on brain activity under a general operation, aiding anaesthetists in keeping patients optimally anaesthetised. The Australian manufactured and designed, electroencephalographically based (EEG-based), BARM(TM) system is configured to efficiently image and display complex information related to the clinically relevant state of the brain. When commercialized the BARM(TM) system will be offered on a stand-alone basis or integrated into leading brand operating room monitors as "plug and play" option.
BARM(TM) has already received TGA approval, Korean MFDS approval, the CE mark and the company has now made application for its FDA approval in the USA.
The BARM(TM) system is protected by five patent families in multiple jurisdictions worldwide consisting of 36 granted patents. Cortical will continue to drive the development of BARM(TM) and maintain its intellectual property.
About BARM(TM)
The BARM(TM) technical approach is different from other medical brain monitoring devices currently available in the market in that its underlying algorithm produces EEG indexes which are directly related to the physiological state of the patient's brain. Such monitoring is gaining significant use during surgery, however even with the use of EEG monitors, it is not uncommon for there to be a critical imbalance between the patient's anaesthetic requirements and the anaesthetic drugs given.
To date, existing EEG based depth of anaesthesia ("D o A") monitors operate in the context of a number of well documented limitations: (i) inability to monitor the analgesic effects; and (ii) reliably measure certain hypnotic agents.
The above limitations highlight the inadequacies in current EEG based depths of anaesthesia monitors particularly given surgical anaesthesia requires both hypnotic and analgesic agents and muscle relaxants.
BARM(TM) Technology and Addressable Market
BARM(TM) is focussed not only on monitoring the inhalation methodology of anaesthesia delivery, but importantly has a very strong focus on Total Intravenous Anaesthesia (TIVA) monitoring. TIVA is a method of inducing and maintaining general anaesthesia without the use of any inhalation agents and is growing in popularity not least because of its use eliminates Greenhouse gases that are a direct consequence of gaseous anaesthesia. TIVA is also a cheaper option. TIVA is becoming more widely accepted, particularly in Europe and approximately 29 million major general surgeries are conducted in the European Union each year, of which 55% (circa 16 million) are balanced anaesthesia (using a combination of intravenous agents such as propofol and volatile gases) and 20% are total intravenous anaesthesia using propofol. This creates a growing market opportunity for BARM(TM) of between US$83m to $229m in the European Union alone.
Additionally, there is growing recognition amongst health governing bodies to recommend the use of brain monitors during operations involving general anaesthesia such as in the UK; "The use of EEG-based depth of anaesthesia monitors has been recommended in patients receiving total intravenous anaesthesia because it is cost effective and because it is not possible to measure end-tidal anaesthetic concentration in this group" (source: nice.org.uk).
Additional Potential applications for BARM(TM) in helping mitigate or reduce Cognitive decline in the elderly after surgery and anaesthesia as a result of brain monitoring A recent editorial in International Psychogeriatrics concluded that 'anaesthesia and surgery induce cognitive dysfunction in susceptible individuals. Susceptible people are thought to include the elderly. This is a serious problem. It was recently estimated that there are annually over 230 million procedures with general anaesthesia worldwide. There are over 880 million people > 60 years old in the world today. The latter figure is predicted to grow rapidly as life expectancy increases, particularly in developing countries.
The consensus statement of the First International Workshop on Anaesthetics and Alzheimer's Disease concluded that 'there is sufficient evidence at multiple levels to warrant further and more definitive investigations of the onset and progression of Alzheimer's disease and neurodegeneration after anesthesia and surgery.
About BPH Energy Limited
 BPH Energy Limited (ASX:BPH) is an Australian Securities Exchange listed company developing biomedical research and technologies within Australian Universities and Hospital Institutes.
BPH Energy Limited (ASX:BPH) is an Australian Securities Exchange listed company developing biomedical research and technologies within Australian Universities and Hospital Institutes.
The company provides early stage funding, project management and commercialisation strategies for a direct collaboration, a spin out company or to secure a license.
BPH provides funding for commercial strategies for proof of concept, research and product development, whilst the institutional partner provides infrastructure and the core scientific expertise.
BPH currently partners with several academic institutions including The Harry Perkins Institute for Medical Research and Swinburne University of Technology (SUT).
| ||
|